Publications of Palmyrah Research Institute,
Sri Lanka 2012-2016
Enhancing the Capacity of Palmyrah Palm (Borassus flabellifer L) through Science
“Touch of Wonder of Palmyrah Realized through Science”
Contents
Publications in International Journals Publications in International conference
Full papers Abstracts
Publications in National conferences Full papers
Abstracts Publications in Newspapers Publications in Book
1. Publications in International Journals
IOSR Journal of Agriculture and Veterinary Science (IOSR-JAVS) 9(6), pp. 51-57, 2016
Microbial, Physico- chemical and Sensory Evaluation of Preserved Palmyrah Fruit Pulp
Robika Kailayalingam, Subajini Mahilrajan*, SriThayalan SriVijeindran and Ponnuchamy Navaratnam
Palmyrah Research Institute, Jaffna, Sri Lanka
ABSTRACT: Fruits of Palmyrah palm (Borassus flabellifer) are seasonal; therefore their fibrous (mesocarp) fruit pulp (PFP) extracted with water and should be preserved with lengthened shelf life to ensure its availability in local and international market throughout the year. Therefore a study on preservation of PFP was carried out with or without various concentrations of preservatives, Sodium benzoate (SB), Sodium metabisulphite (SMS) and combinations of the both at different ratio. pH of the PFP was adjusted to 3.8 with citric acid, heated in a water bath at 90oC for 20 Sec, preservatives were added, mixed well then bottled pulp was heated at 80oC for 30 min in thermostatic water bath and kept at room temperature (30oC) for 180 days. Initial pH with stabilization has come to about 4.2. Aliquots of them were withdrawn periodically (at 30days intervals) and were analyzed for microbial, physicochemical and sensory characteristics. PFP alone (without preservatives) was spoiled with increasing pH by showing adverse characteristics (unacceptable odour) before 24hours of storage. All the treatment showed significant (p<0.001) increased in total soluble solid (10.82 – 13.10 obrix) and declined in pH (4.42 – 4.14) was observed with a proportional increase in the acidity (0.71- 0.91%) for treatments of T1 – T5 (containing SB), T6 –T10 (containing SMS) and T11 – T15 (containing both SMS & SB) up to 180 days. But no colony (Total Plate Count) was observed in the pulp treated with SMS and with combination of SMS & SB at various concentrations up to 120 days of storage. Among the all treatments the pulp treated with SB were found to be inferior in both colour and flavour characteristics. Even though it was found that PFP treated with SMS, T6 –T10 could be stored for extended period of 180 days without any major changes in chemical,
microbiological and sensory characteristics, whereas T7 (with SMS,0.4g/l ) was selected as the best treatment based on the overall acceptability.
Keywords: Palmyrah Fruit Pulp, preservatives, sensory evaluation
1. Introduction
Palmyrah (B.flabellifer) fruit is the oldest and most important tropical fruit. It is indigenous or naturalized throughout tropical and subtropical South and Southeast Asia. Palmyrah fruit is mostly used as fresh fruit for pinattu (Dried pulp) and oil cakes, but due to its perishable nature it cannot be stored for long period of time. Pulp is yellow in colour due to the presence of carotenoids (Provitamin A). It is a good source of vitamin C and contains appreciable amount of pectin [1]. Jeyaratnam (1986) [2] said that pulp contains appreciable amount of saponin and also believed that pulp provides dermatitis relief.
During peak of harvest season (Aug- Oct) large quantity of fruits are wasted due to limited shelf life in storage. In order to make the PFP available during the off season it has to be preserved with lengthened shelf life. Sales centres of Palmyrah Development Board, Katpakams sell bottled PFP to prepare fruit base edible products. But during storage period colour of the bottled pulp turns to yellowish brown. Despite the fact it has to be developed with favourable chemical treatment for the preservation of PFP. Because of its high fermentable nature under the influence of microbes, it is dried as Pinattu for short term preservation. But it is also preserved by making panampanam (diluted drink), cordial, crush and jam with moderate shelf life.
Sodium benzoate (SB) and potassium metabisulphite (PMS) are commonly used as preservatives for long term storage of fruit pulp because of their better antimicrobial activity [3]. The maximum level for the use of these chemicals in fruit preservation including pulp and purees as described in the Codex Standards adopted in 2001 and 2006 are 1000 mg/kg SB as benzoic acid and 500 mg/kg PMS as residual SO2 [4]. Keeping in view these facts, this study was undertaken to find out the inhibitory effect of SB, SMS and both in different ratio with varying concentrations for microbial, chemical, physical and sensory quality of PFP stored at room temperature.
The aim of this study was to extend the shelf-life of the PFP by determining the best proportions of food additives like sodium benzoate and sodium metabisulphite (SB, SMS
and both in combination) to be applied for preservation of PFP at room temperature (30ºC). If storage of pulp can be improved for a long period, both PFP and its based food products will increase earnings in the Sri Lanka domestic and foreign markets.
2. Material and methods
This research was approved by Research and Development division of Palmyrah Development Board.
2.1 Determination of Microbial Count
The method of Sri Lankan Standard: 516 Part 1: 1991 [5] was used.
Preparation of Nutrient Agar Plates
Plate Count Agar (PCA) HIMEDIA Laboratory Pvt. Ltd medium (2.35g) in a 250ml conical flask was dissolved in 40ml of distilled water by heating in a water bath, made up the volume to 100ml with the same, plugged with cotton wool well, sterilized at 121oC and 15lb in-2 pressure for 15 min and then allowed to cool to 45oC.
Serial dilution
Sample (10g) was transferred into a labeled sterile dilution bottle, made up the volume to 100ml with peptone (HIMEDIA) water (peptone 1g, NaCl (HIMEDIA) 8.5g made up to1000ml with water) under the sterile condition. Aliquots of it were taken after the thorough mixing by using vortex mixer (VELP SCIENTIFICA ZX3) and repeated the same process to obtain required dilution.
Microbial Count
Diluted sample (1ml) was transferred into each sterile plate. 20ml portion of the medium was poured to each plate in the laminar flow chamber (BIOBASE), mixed gently and allowed it to cool at room temperature. Plates of different dilutions were incubated at 37°C for 48hrs and the appeared colonies in the plates were counted and the total colonies were calculated. This experiment was repeated twice and the mean values (CFU/g) of these were calculated.
2.2 Physicochemical analysis
Total Soluble Solids (TSS)
Total soluble solids (TSS) of each sample were determined directly by using Refractometer (HSR500, Japan) at room temperature and expressed in terms of oBrix value.
Acidity
The method of SLS: 730:2010 [6] was used. The acidity of the given sample was determined as citric acid (%w/w) by titrating 10ml of sample against 0.1 N NaOH (SIGMA) using phenolphthalein (SIGMA) as an indicator.
pH
Homogenized sample (25ml) was taken in a clean beaker (25ml) and its pH was measured by using a digital pH meter (Sension PH 31-Spain) at room temperature.
2.3 Sensory evaluation
The method described by Larmond (1977) [7] was used. Selected sample was evaluated by a panel of judges from Palmyrah Research Institute staff with Oral Consent Scripts for sensory characteristics like colour, flavour, texture, mouth feel and over all acceptability. The judges were provided with prescribed questionnaires to record their observation. The information contained on the performance was 5 = Like very much; 4 = Like slightly; 3 = Neither like nor dislike; 2 = Dislike slightly; 1 = Dislike very much. The panelists expectorated the sample and rinsed mouth using distilled water between samples.
2.4 Preparation of PFP
Well ripened Palmyrah fruits available in plenty in their season at Kaithady, Northern Region of SriLanka, were washed twice with potable water and their tepals (tops) were removed then again washed with potable water and dipped in hot water for few seconds. Then ectocarp (skin) was peeled manually, the remainder (nutlets) was macerated with warm water (nutlet: water in ml = 1:100). Diluted pulp (PFP) was extracted manually using sieve after the removal of seeds and insoluble fibres.
Adjustment of pH
Initial pH of the PFP was measured with pH meter (Sension+ PH 31-Spain) and then the pH of the pulp was adjusted to proper pH 3.8 with concentrated solution of food grade commercially available citric acid and mixed well.
Blending
Acidified pulp was blended by using electric blender at low speed for 5 min.
2.5. Effect of heating at 80oC for 30min Pasteurization in the preservation of PFP PFP (pH3.8) poured into the capped clear glass bottle (100ml, without the addition of preservatives) was heated in a thermostatic water bath at 80oC for 30 minutes and then allowed to cool to room temperature [8,9] and stored for a period of 150 days. Aliquots of them were taken for the analysis.
2.6 Effect of preservatives in the preservation of PFP
Common preservative Sodium Benzoate and Sodium metabisulphate (Food Grade) available in local market were used. The PFP was heated in a thermostatic water bath GEMMYCO at 90oC for 20 sec., preservatives were added according to the TABLE 1, mixed well and they were transferred into clear sterile glass bottles separately and capped well. The bottled pulp was heated in a thermostatic water bath at 80oC for 30 min and then allowed to cool to room temperature [8,9]. They were stored at room temperature for a period of 180 days. Aliquots of them were taken in 30 days interval for the analysis.
Statistical analysis
Results obtained from chemical analysis (pH, brix and acidity) with three replicate were subjected to three way ANOVA. The significant difference among the treatments was tested in Least Significant Difference (LSD) at 5 % level of significance using SAS (version
9) System software.
Friedman non-parametric statistical method was used to analyze the sensory evaluation data based on 5-point hedonic scales. In this data analysis 95% confidence interval was considered, and analysis was done using Minitab 13 software.
Table 1: Concentrations of different preservatives used in preservation of PFP, alone or their combination at varying ratio
| Treatments | SB%, (w/v) | SMS%, (w/v) |
| T1 | 0.04 | – |
| T2 | 0.08 | – |
| T3 | 0.12 | – |
| T4 | 0.16 | – |
| T5 | 0.2 | – |
| T6 | – | 0.03 |
| T7 | – | 0.04 |
| T8 | – | 0.05 |
| T9 | – | 0.06 |
| T10 | – | 0.07 |
**Half of the concentration of each preservative used alone before was used together here.
1. Results and Discussion
Palmyrah fruit and their products have gained considerable importance by contributing significantly to the economy of Sri Lanka. On the other hand freshly extracted pulp is highly attractive in appearance and possesses good taste and aroma, but it deteriorates rapidly in 24h. This is mainly due to fermentation caused by moulds, yeasts and bacteria. The enzymes secreted by them may affect the colour and flavour adversely. Chemicals present in the pulp may react with one another and spoil its taste and aroma. Air coming in contact with the product may react with the glucosidal substances present in it. This deterioration must be avoided by application of the food preservation principle which first involves the prevention or delay of the microbial spoilage.
The present study was carried out to identify a suitable chemical preservative/s such as sodium benzoate, sodium metabisulphate either alone or in combination for satisfactory storage of PFP at room temperature. Efficiency of preservation and storage behavior of fruit pulp is depended on physicochemical characteristics such as acidity, pH and Total Soluble Solids (TSS) and biological parameters. Period of storage had shown a pronounced effect on physicochemical attributes of chemically preserved PFP.
1.1 Microbiological evaluation
Benzoic acid inhibits the growth of mold, yeast [10] and bacteria. It is either added directly or created from reactions with its sodium, potassium or calcium salt. The mechanism starts with the absorption of benzoic acid into the cell. If the intracellular pH changes to 5 or lower, the anaerobic fermentation of glucose through phosphofructokinase is decreased by 95%. The efficiency of benzoic acid or benzoate is thus depended on the pH of the food [11]. Sodium metabisulphite releases SO2 gas when added to water, SO2 kills yeasts, fungi and some bacteria and also it acts as an antioxidant.
.
Microbial analysis of fresh PFP showed that total palate count (TPC) at the initial time was 2×106cfu/g and also heat treated PFP at 800C (without the addition of chemical preservatives) was spoiled before 15 days whereas the pulp containing preservatives (T1- T15) exhibited no microbial growth up to 120 days period of storage. At 150 days of storage, the mean TPC was significantly increased from 0 to 9cfu/g for T5 to T1. A maximum mean value was recorded in T2 while minimum value was observed in T4. Treatments T6-T15 showed growth of microorganism at 180 days of storage but counts (cfu/g) were in the acceptable range given in SLS 730: 2010. Hence chemical preservatives decreased the microbial load significantly in PFP. These results are in accordance with the findings reported by Hussain et al., (2003) [12] and Hashmi et al., (2007) [13] for mango pulp.
3.2. Physicochemical evaluation
3.2.1. Acidity and pH
There were interaction between preservatives, concentrations and storage period for acidity values while except preservatives for pH. Significantly higher mean pH was observed for PFP treated with SB (4.32) when compared with SMS (4.24) and both SB , SMS (4.21) and there were significant different (p<0.05) between mean pH of the pulp with the storage period while which was decreased with period of storage. PFP treated with SMS (T6-T10) and both SB, SMS (T11-T15) showed less increase in pH compared with PFP treated with SB (T1-T5).This may be due to either utilization or neutralization of acidic compounds present in the pulp otherwise compound/s secreted by organism. This condition may facilitate organism/s to prolong their growth and thereby leads to deteriorate the pulp. Abbassi et al., (2009) [14] attributed the increase in pH and the decrease in titrable acidity with increased storage period of the mangoes.
The results relating to the increase in acidity and decrease in pH (Figure 1) during the storage of PFP are in complete agreement with other researchers [15]. pH plays dual role in the fruit juices by acting as a flavour promotion and preservation. Decrease in pH of the fruit pulp samples proportional to increase in acidity has been confirmed by several researchers and may be attributed to the presence of SB in the pulp samples [16, 17].
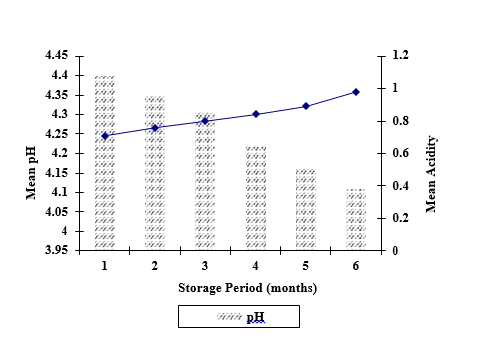
the period of storage
Fig 1: Change in pH and acidity of PFP incorporated with preservatives during the period of storage
Significantly higher mean acidity was observed for PFP treated with SB (0.88%) while no significance difference between pulp with SMS (0.79%) and both SB, SMS (0.80%).Change in acidity of PFP with the period of storage has been showed in Figure 1 while there were no significant different (p<0.05) between 60, 90 and 120 days of the storage. While that acidity was significantly increased from 30-180 days. PFP treated with SMS (T6-T10) and both SB, SMS (T11-T15) showed less increase in percentage of acidity compared with PFP treated with SB (T1-T5).The increase in acidity may be ascribed to rise in the concentration of weakly ionized acid and their salts during storage and also due to formation of acid by degradation of polysaccharides and oxidation of reducing sugars or by breakdown of pectin substances and uronic acid [19, 17]
3.2.2 Total Soluble Solids (TSS)
There were interaction between all factors such as preservatives, concentrations and storage period. Amin et al., (2008) reported the effect of time of fruit harvest affects the fruit quality. The variability in TSS in the PFP might be attributed to the alteration occurring in cell wall structure during ripening process. Moreover, various hydrolytic enzymes also affect complex carbohydrates changing them into smaller compounds. Significantly higher mean obrix was observed for PFP treated with both SB, SMS (13.00) when compared with SMS (11.02) and SB (12.15). TSS was significantly increased gradually up to a storage
period of 180 days (TABLE 2). While there were no significant difference between 60 and 60 also 120 and 150 days of storage. PFP treated with both SB, SMS (T11-T15) and SB (T1-T5) showed more increase in obrix compared with PFP treated with SMS (T6-T10) while there was significance different between treatments.
Table 2: Effect of storage on TSS of the PFP (oBrix)
| Treatment s | Storage (days) | |||||||
| 30 | 60 | 90 | 120 | 150 | 180 | Mea n | ±SD | |
| T1 | 11.83 | 11.84 | 11.89 | 11.95 | 11.90 | 12.00 | 11.9 | 0.06 |
| T2 | 12.2 | 11.96 | 12.01 | 11.98 | 11.90 | 12.00 | 12.01 | 0.09 |
| T3 | 12.17 | 12.03 | 12.11 | 12.48 | 12.25 | 12.50 | 12.26 | 0.18 |
| T4 | 12.26 | 12.13 | 12.15 | 12.5 | 12.35 | 12.60 | 12.33 | 0.17 |
| T5 | 12.43 | 12.17 | 12.19 | 12.38 | 12.25 | 12.30 | 12.29 | 0.09 |
| T6 | 10.79 | 10.93 | 10.97 | 10.95 | 10.98 | 11.00 | 10.94 | 0.07 |
| T7 | 10.86 | 10.90 | 10.93 | 10.98 | 10.95 | 11.00 | 10.94 | 0.05 |
| T8 | 10.77 | 10.83 | 10.9 | 10.89 | 10.78 | 10.80 | 10.83 | 0.05 |
| T9 | 10.86 | 10.92 | 11.26 | 11.45 | 11.40 | 11.50 | 11.23 | 0.25 |
| T10 | 10.77 | 10.83 | 11.09 | 11.45 | 11.40 | 11.50 | 11.17 | 0.29 |
| T11 | 12.65 | 12.9 | 12.91 | 12.95 | 12.90 | 13.20 | 12.92 | 0.16 |
| T12 | 12.84 | 12.85 | 12.87 | 12.90 | 12.85 | 13.20 | 12.92 | 0.13 |
| T13 | 12.86 | 13.03 | 12.29 | 13.20 | 12.95 | 13.60 | 12.99 | 0.39 |
| T14 | 12.77 | 12.85 | 13.00 | 13.40 | 12.99 | 13.60 | 13.10 | 0.3 |
| T15 | 12.71 | 12.78 | 13.01 | 13.30 | 13.20 | 13.60 | 13.10 | 0.31 |
| Mean | 11.92 d | 11.93d | 11.97c | 12.18b | 12.07b | 12.29a | ||
| ±SD | 0.86 | 0.85 | 0.78 | 0.88 | 0.82 | 1.00 |
Each value in the table is represented as mean ± SD (n = 3). Values in the mean row followed by a different letters (a-d) are significantly different (p< 0.05).
About half of the soluble sugars of PFP are mainly composed of fructose (3.4%), with about 6.6% sucrose and 3.5% glucose. The high sugar content of pulps from ripe fruits might be attributed to the transformation of starch into soluble sugars under the action of phosphorylase enzyme during ripening [19, 20] and water soluble pectin from insoluble proto pectin in lime squash and fruit bases, respectively [21, 22].
3.3 Sensory evaluation
Every fruit is selected by its visual appearance because colour of fruit is main attribute for judging the eatable quality of fruit and the same process is applied for the colour of PFP in this research. The values for colour of all the treated samples decreased during storage at ambient temperature. The PFP from various varieties collected from different production sites were not exactly at the similar ripening stage thus they may vary in colour and other sensory characteristics. Aina & Oladunjoye (1993) [23] reported that the colour change in mangoes is primarily associated with several biochemical changes, both degradation and synthesis of various classes of molecules including carotenoids in fruit.
A number of biochemical reactions or metabolic activities are involved in the ripening process of mango fruit such as increased respiration, ethylene production, change in structural polysaccharides causing softening, degradation of chlorophyll and synthesis of carotenoids, changes in carbohydrates or starch conversion into sugars, organic acids, lipids, phenolic compounds and a number of volatile compounds. All these changes lead to ripening of fruit with softening of texture to acceptable quality. These factors predominantly contribute towards developing a total sensory profile of the mango fruit [24].
The colour of T2 and T3 was spoiled and turned yellowish brown during 90 days of storage interval. Median values of colour score for T7 and T8 is high (44.5) when compared with other treatments while T3 showed very less score of median and also this median value decreased with increase concentration of preservatives (TABLE 3). Flavour is comprised of aroma and taste. The score for flavour decreased for PFP during storage at room temperature. Flavour score of T7 and T8 were higher than that of T12 and T13during 180 days of storage while the scores noted for T2 and T3 were very less. Score of overall acceptability for T2 and T3 was less than other treatments when compared with others and median overall acceptability score at initial time of storage was highest for T7 (Figure 2).
Table 3: Effect of selected treatments on median value of sensory analysis at 180 days of Storage
| Flavour | Colour | Mouth feel | Texture | Overall acceptability | |
| T2 | 14.5 | 14.5 | 17 | 19.5 | 15 |
| T3 | 15.5 | 13 | 17 | 19.5 | 13 |
| T7 | 42 | 44.5 | 37.5 | 31.5 | 37.5 |
| T8 | 42 | 44.5 | 40.5 | 36.5 | 30 |
| T12 | 37.5 | 34 | 39 | 41 | 33.5 |
| T13 | 37.5 | 38.5 | 38 | 41 | 32.45 |
Organic acid and sugars ratio primarily creates a sense of taste which is perceived by specialized taste buds of the tongue. Thus, sweetness due to sugar and sourness from organic acids are dominant components in the mouth feel of many fruits [25]. But in PFP mouth feel is due to bitter compounds called flabelliferins which vary with many factors such as place at which palmyrah tree is grown, type of fruit and stage of ripening at which that fruit is tested. These factors play a major role in the assessment of its sensory qualities and acceptability [26]. In this study, T7 had highest overall acceptability at initial and 180 days of storage therefore based on the sensory characteristics the T7 was recognized as relatively better than the selected treatments (Figure 2).
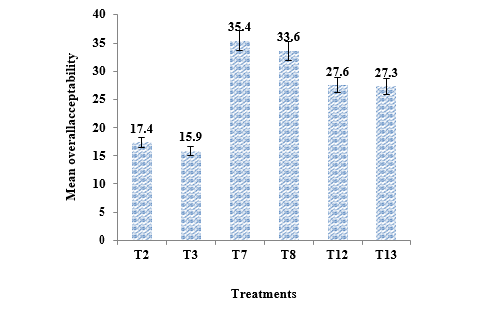
1. Conclusion
From this research, it is evident that storage of PFP incorporated with preservatives showed an increase in acidity and brix values besides the decreased level of microflora with time. However, according to the organoleptic evaluation done up to 180 days of period of storage PFP containing SB was rejected by panelists, whereas among the PFP containing SMS alone and combination of SB & SMS, PFP with SMS (0.4 g/l) was selected as better with respect to overall acceptability. Hence it is proved that pasteurization of PFP incorporated with SMS (0.4 g/l) at pH3.8 and 80oC for 30min is needed to store PFP for 6months without any loss of acceptable characteristics.
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
PN- made consultancy and revising the manuscript; RK carried out the research activities and revising the manuscript; SM carried out the research activities, statistical analysis and drafted the manuscript; SSV- coordinated & management of research activities. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Ministry of Traditional Industries and Small Enterprise Development; Sri Lanka for the financial support and also would like to express their gratitude to all the staff of Palmyrah Research Institute, Jaffna, Sri Lanka, for their kind support and assistance in the project.
References
- Jansz ER, Wickremasekara NT and Sumuduni KAV, A Review of the Chemistry and Biochemistry of seed shoot flour and fruit pulp of the Palmyrah palm (Borassus flabellifer L.). Journal of National Science Foundation of Sri Lanka. 2002; 30(1&2): 61-87.
- Jeyaratnam M, Studies on the Chemistry and Biochemistry of palmyrah products. M.Phil. Thesis. University of Jaffna, Sri Lanka. 1986; 1-200
- Manganelli E. and Casolari A, Sensitivity of yeasts to sorbic and benzoic acids and their salts. Ind Conserve, 1983; 58: 23-25.
- Akhtar s, Riaz1 M, Ahmad A, Nisar A, Physico-chemical, microbiological and sensory Stability of chemically preserved mango pulp. Pak. J. Bot. 2010; 42(2): 853-862.
- SLS 516: 1991, Microbiological analysis total plate count, Sri Lanka Standards Institution, 1991, 12.
- SLS 730: 2010, Ready to serve fruit drinks, Sri Lanka Standards Institution, 2010, 8-20.
- Larmond E, Laboratory methods of sensory evaluation of foods. Publication 1673. Canada; Deptt. of Agric Ottawa; 1977.
- Senesi E, Torreggiani D and Berstoalo G Effect of pasteurization on the quality of osmodehydrated fruits: Cling-stone peaches and sweet cherries. Food Sci. Technol.1988; 63(4), 358-363.
- Kirk RS and Sawyer, Pearson’s Chemical Analysis of Food, 9th ed. Churchill Livingstone Inc. New York; USA; 1991.
- Warth AD, Mechanism of action of benzoic acid on Zygosaccharomycesbailii: effects on glycolytic metabolite levels, energy production, and intracellular pH. Appl. Environ. Microbiol, 1991; 57 (12): 3410–4.
- Pastrorova I, de Koster CG and Boom JJ, Analytic Study of Free and Ester Bound Benzoic and Cinnamic Acids of Gum Benzoin Resins by GC-MS HPLC-frit FAB-MS. Phytochem. Anal, 1997;8 (2): 63–73.
- Hussain S, Rehman S, Randhawa MA and Iqbal M, Studies on physico-chemical, microbiological and sensory evaluation of mango pulp storage with chemical preservatives.
J. Res. (Sci.), BZ Uni. Multan. Pak. 2003; 14: 1-9.
- Hashmi MS, Alam S, Riaz A and Shah AS, Studies on microbial and sensory quality of mango pulp storage with chemical preservatives. Pak. J. Nutr, 2007; 6: 85-88.
- Abbasi NA, Zafar I, Maqbool M, Hafiz IA, Post-harvest quality of mango (Mangifera indica L.) fruit as affected by chitosan coating. Pak J of Bot. 2009; 41(1): 343- 357.
- Doreyappy-Gowda LND and Huddar AG, Studies on ripening changes in mango (Mangifera indica L.) fruits. J. Food Sci Tech Mysore, 2001;;38: 135-137.
- Bajwa EE, Naeem Z Anjum J and Nazir A, Development, standardization and storage studies on watermelon-lemon. Pak. J. Food Sci, 2003; 12: 21-24.
- Hussain I, Zeb A, Shakir I and Shah AS, Combined effect of potassium sorbate and sodium benzoate on individual and blended juices of apricot and apple fruits grown in Azad Jammu and Kashmir. Pak. J. Nutr, 2008; 7(1): 181-185.
- Iqbal SA, Yasmin S.Wadud and Shah WH, Production storage packing and quality evaluation of Gouva Nectar. Pak. J. Food Sci, 2001; 11: 33-36.
- Germain P and Linden G,. Activités enzymatiques. In: Analyse des constituants alimentaires. (Eds.): B. Deymier, J.L. Multon and D. Simon. Techniques d’Analyse et de contrôle dans les industries agroalimentaires,Tec. et Doc Lavoisier, Paris, 4; 211-244,1981.
- Favier JC, Ireland RJ, Laussuc C and Feinberg M, Répertoire général des aliments. Tome 3: Table de composition des fruits exotiques, fruits de cueillette d’Afrique. INRA (Ed.), 1993;. 55-59.
- Khalil M, Ramzan M, Ali A and Riaz RA, “Studies on the preparation and storage stability of comminuted lime squash”, Pak. J. Sci. Ind. Res., 1979; 22 (5), 267-272.
- Riaz RA, Ali A and Saleem M, Studies on the preparation and storage stability of communited Kinnow fruit beverage bases. Pak. J. Sci. Ind. Res., 1988; 32 (8), 574-578.
- Aina JO, Oladunjoye OO, Respiration, proteolytic activity and textural changes in ripening African Mango (Irvingiagobenesis) fruit. J of Sci of Food and Agri. 1993; 63: 451- 454.
- Herianus JD, Singh LZ and Tan SC, Aroma volatiles production during fruit ripening of ‘Kensington Pride’ mango. Post-Harvest Biol. Tec. 2003; 27: 323-336.
- Kays SJ, Post-harvest physiology of perishable plant products Vas Nostrand Rein Hold Book, AVI Publishing Co, 1991; 149-316.
- Mtebe K, Mamiro P and Fweja L, Sensory attributes, microbial quality and aroma profiles of off vine ripened mango (Mangifera indica L.) fruit. African Journal of Biotechnology, 2006; 5: 201-205.
International Journal of Scientific Research in Agricultural Sciences,
3(3), pp. 062-072, 2016
Comparative Study on Phytochemical and Antimicrobial Activity of
Different Solvent Extracts of Pinattu
Srikantharasa Srishankar1, Subajini Mahilrajan*2, W.A.J.P. Wijesinghe 1 and Srithayalan Srivijeindran2
1 Uva Wellassa University, Sri Lanka
2Palmyrah Research Institute, Jaffna, Sri Lanka
Abstract
Palmyrah (Borassus flabellifer) fruit is mostly used as fresh fruit, because of its perishable nature it is traditionally preserved as dried fruit pulp as pinattu and constituents of crude extracts of pinattu were evaluated. Samples were collected from three different branches of Palmyrah Development Board and extracted with different solvents as aqueous, methanol, ethyl acetate and petroleum ether then concentrated extracts were used for the study. Alkaloids and tannins were not detectable in all the extracts while aqueous and methanol extracts gave positive results for carbohydrates, proteins, phytosterols, saponins, flavonoids, phenols and amino acids and fats and fixed oils. Spectroscopic determination of total phenolic, flavonoids and saponin content were significantly (p<0.05) different among the solvents and the highest amount was identified, in aqueous extract (19.92±0.5)mg/g, methanol extract (0.18±0.0)mg/g and methanol extract (509.88±4.18)mg/g respectively. Based on their diameter of the zone of inhibition least MIC of aqueous extract for Staphylococcus (1.4±0.1cm), E.coli (1.2±0.0cm), Pseudomonas (1±0.0cm) and methanol extract for E.coli (1.4±0.1cm) and Pseudomonas (1.1±0.00cm) was 0.5mg/ml. The Salmonella and Bacillus was showed 0.75mg/ml for aqueous extract while 0.25 and 1.0mg/ml for methanolic extract respectively. Klebsiella was showed 0.25mg/ml for both aqueous extract (1.1±0.0cm) and methanol extract (1.35±0.0cm). Highest inhibition zone was observed for 1 mg/ml of aqueous extract (2.15±0.2cm) in Protease when compared with positive control (1.9±0.1cm).This finding shows that crude aqueous and methanol extract of pinattu contains high amount of phytochemicals, exhibit significant antibacterial activity with relatively lower MIC (≤ 1mg/ml) when compared with ethyl acetate and petroleum extracts.
Key words: Antibacterial activity, Pinattu, Phytochemicals, Saponin and Solvents
1. INTRODUCTION
Total phenol, total saponin and the total flavonoids content determination in palmyrah dried fruit pulp leather (pinattu) extracts is interesting helpfulness for global nutritionists due to their valuable effects on human and animal health. Thus, investigation of various phytochemicals present in palmyrah based product is the noble study in order to understand their health benefits.
Phenolics are one of the key secondary metabolites existing in the plant kingdom. They are low molecular weight compounds (mol. wt. <2000 amu) universally present in all tissues of higher plants and play an important role during the development of a plant. They have multiple biological effects, including antioxidant activity (Gulcin et al. 2005), anti- inflammatory activities and metal chelation properties (Rice-Evans et al. 1997).
Flavonoids the most common group of polyphenolic that are found universally in plants. These are widely distributed in plant achieving many functions. They are important in plant for normal growth development and defense against contamination and injury (Kähkönen et al. 1999), anti-aging (Hadnick et al. 1998), antioxidant (Croteau et al. 2000), antibacterial and antifungal activities (Hassan, 2010), anticancer, anti-cardiovascular disease and anti- inflammatory (Nijveldt et al. 2001).
Saponins are among several plant compounds which have beneficial effects. Among the various biological effects of saponins are antibacterial, anticancer (Mathers, 2002) and antiprotozoal (Avato, 2006). Saponins are surface-active glycosides with detergent, wetting, emulsifying, and foaming properties (Mitra and Dungan, 1997). The palmyrah fruit pulp contains pectin, sugar, carotenoids in addition to numerous steroidal saponins (flabelliferins) (Thabrew and Jansz, 2004). This was suspected to reduce weight gain in ICR mice (Ariyasena et al. 2002) and also inhibit the increase in blood glucose after a glucose intake, while improving the level of faecal glucose, thus suggesting an inhibition of intestinal glucose uptake (Uluwaduge et al. 2005).
Nowadays antibiotics are effectiveness against serious bacterial infections. However, only one third of the infectious diseases known have been treated from these synthetic products. This is because of the emergence of resistant pathogens due to the indiscriminate use, incessant and misuse of antibiotics. One of the methods to reduce the resistance to antibiotics is by using antibiotic resistance inhibitors from plants. Plants are known to
produce a variety of compounds to defend themselves against a variety of pathogens. It is expected that plant extracts showing target sites other than those used by antibiotics will be active against drug resistant pathogens (Sen and Batra, 2012). Palmyrah palm have been used as traditional treatments for numerous human. Hence, researchers have recently paid attention on biologically active compounds, isolated from plant species used in herbal medicines for the development of novel drugs and functional foods.
Although various studies have been reported on various plant extracts, while there was little research has been conducted on palmyrah palm. Therefore, this study was conducted to determine the amount of the total phenol, flavonoid and saponin content by using spectroscopic methods and antimicrobial activity of various extracts of palmyrah dried fruit pulp leather (pinattu).
2. MATERIALS AND METHODS
This research proposal was approved by Research and Development division of Palmyrah Development Board and research committee of Uva Wellassa University (6.9826° N, 81.0768° E), Sri Lanka.
2.1 Collection of sample
Palmyrah fruit pulp leather was obtained from the three different branches of Palmyrah Development Board (PDB) then cut into small species and pool together. After that 100 g of sample was weighted in random manner.
2.2 Preparation of plant extracts
Palmyrah fruit pulp leather (100 g) was extracted in a soxhlet extractor for 24 hours with petroleum ether (boiling point 40-60oC, polarity index: 0.1), ethyl acetate (boiling point 76.5oC, polarity index: 4.3), methanol (boiling point 65oC, polarity index: 6.6) and water (polarity index: 9) separately based on polarity index. The extracts were evaporated under reduced pressure using rotatory evaporator (IKA), then extracts stored at 4 °C (Gulcin, 2005). All the extractions were performed in duplicate.
2.3 Qualitative evaluation of phytochemicals
Petroleum ether, ethyl acetate, aqueous and methanol extracts were tested for the presence of phytochemical constituents by performing the standard methods such as Mayer’s test, Dragen-dorff’s test and Wagner’s test for Alkaloids, Molisch’s test and Fehling’s test for
carbohydrates, foam test for saponins , Libermann-Burchard test and Salkowski’s test phytosterols, ferric chloride test for phenols., alkaline reagent test and lead acetate test for flavonoids, Ninhydrin test and Xanthoproteic test for protein and aminiacids, gelatin test for tannins, modified Borntrager’s test for glycosides and test for fat and oil.
2.4 Determination of total phenolic content
The total phenolic content of the pinattu extracts was determined using the Folin- Ciocalteu reagent (Maurya and Singh, 2010). The reaction mixture contained 0.5ml of diluted extracts, 2.5ml of freshly prepared 10 % diluted Folin-Ciocalteu reagent and 2 ml of 7.5% sodium carbonate. Mixtures were kept at ambient conditions for 30 min to complete the reaction. The absorbance at 760 nm was measured. Gallic acid was used as standard and the results were expressed as mg gallic acid (GAE)/g pinattu.
2.5 Determination of total flavonoid content
Total flavonoid content was determined using aluminium chloride (AlCl3) according to a known method (Ordonez et al. 2006) using quercetin as a standard. The plant extract (3 ml) was added to 5% NaNO2 (0.3 ml). After 5 min at room temperature, AlCl3 (0.3 ml, 10%) was added. After further 5 min, the reaction mixture was treated with 2 ml of 1 M NaOH and the absorbance was measured at 510 nm. The results were expressed as mg quercetin (QE)/g pinattu.
2.6 Determination of total saponin content
Total saponins contents in pinattu extracts were estimated (Hiai et al. 1976). Different extracts of 0.25ml of solution was taken and 0.25 ml of vanillin reagent (8%, w/v) was added. Then 2.5 ml of 72% (v/v) sulphuric acid was added slowly on the inner side of the wall. After mixing the content tubes were kept in a water bath at 60oC for 10 min then cooled in ice-cold water bath for 4min. Absorbance was measured at 544 nm using spectrophotometer against the reagent blank. Quillaja saponin was used as a standard and the content of total saponins was expressed as mg Quillaja saponin(QS) equivalents /g pinattu.
Statistical analysis
The results obtained from the four extracts with three replicate were subjected to analysis of variance by complete randomized design (CRD). The significant difference among the
extracts was tested in Least Significant Difference (LSD) at 5 % level of significance using SAS software.
3. RESULTS
- Qualitative evaluation of phytochemicals
Phytochemical screening in pinattu (Figure: 1) showed that all the selected tests gave positive results for any of the crude solvent extracts except tannin test (Table 1).
3.2 Quantitative evaluation of phytochemicals
Aqueous, methanol, ethyl acetate, and petroleum ether extracts were prepared to examine the total phenolic, flavonoid and total saponin content of the dried palmyrah fruit pulp by using spectrophotometry.
Total phenolic content was estimated by using Folin-Ciocalteu reagent. Total phenolic content of pinattu was dependent on different solvent extracts and expressed as milligrams of gallic acid equivalents (GAE) equivalent. Table summarizes that total phenolic compounds in solvent extracts varied widely, ranging from 0.011 and 19.92±0.42 mg/g expressed as gallic acid equivalents (GAE). Aqueous extract [19.92(±0.42)] exhibited significantly (p<0.05) highest total phenolic content than methanolic extract [5.74(±0.06)mg/g]. There were no significant different between the extracts of ethyl acetate and petroleum ether. The amount of total phenol obtained from these extract was very less when compared with aqueous extract.
The content of flavonoid expressed asquercetin equivalents. Pinattu extract obtained from water not quantifiable while methanol extract showed 0.18±0.00 mg/g of flavonoid.
Total saponin contents in pinattu (% w/w) expressed as Quillaja saponin equivalents. Which was significantly different with various extracts such as water, methanol, ethyl acetate and petroleum ether was 427.08(±7.84), 509.88(±4.18), 16.62(±0.39) and 34.08(±0.86) mg/g respectively. Methanol extract gave highest saponin content among the selected extract.
3.3 Antibacterial analysis
In the trail experiment of antibacterial activity assay, ethyl acetate extract of pinattu was not exhibited inhibition zone for all tested bacteria. It may be due to the low concentration
of the extract in the solvent. Therefore extracts of aqueous, methanol and petroleum ether obtained from pinattu was selected for antibacterial assay studies.
According to the two-way ANOVA results obtained for all test organisms showed p< 0.001 for both extract and concentration. Hence the main effects were highly significant at 99.9% probability level. Since p value for the interaction effect was found as< 0.001 so there is a significant interaction effect between extracts and concentrations showed at 99.9%
level.
According to the Table 3 all the test bacteria gave highest inhibition zone diameter for aqueous extract except Staphylococcus and Salmonella while these two bacteria showed highest inhibition zone diameter for methanol extract in the meantime petroleum ether extracts showed lowest diameter for all the tests bacteria.
The antibacterial activity of both extracts with different concentration was evaluated according to their diameter of the zone of inhibition against various bacteria and the results were compared with the activity of the standard (chloramphenicol (0.1mg/ml) and solvent, serve as positive and negative control respectively.
Least MIC of Staphylococcus was 0.5 mg/ml for aqueous extract however 0.25 mg/ml for methanol extract (Table 5). Inhibition zone of Staphylococcus was showed no significant different between 1mg/ml concentration of aqueous (1.85±0.07 cm) and methanol (1.85±
- cm) extracts. Zone of inhibition from Staphylococcus in the concentration of 0.75 and
0.5 mg/ml methanolic extract were significantly higher than that of 0.5 mg/ml of aqueous extract. E.coli was showed same least MIC (0.5 mg/ml) for both aqueous and methanol extract. Inhibition zone of E.coli was showed significant different between the concentrations (1.0 mg/ml, 0.75 mg/ml, 0.5 mg/ml, 0.25 mg/ml) of aqueous and methanol extracts. Least MIC of Pseudomonas was 0.5 mg/ml for aqueous extract though 0.5 mg/ml for methanolic extract (Figure 2). Inhibition zone of Pseudomonas was showed no significant different between the positive and 1mg/ml [2.15± (0.07)] cm concentrations of aqueous extracts and 0.75 mg/ml and 1mg/ml [1.7± (0.00)] concentrations of methanolic extracts. Zone of inhibition for Salmonella were showed no significant different between the 1mg/ml concentration of aqueous [1.5± (0.00)] cm and 0.5mg/ml methanolic [1.5± (0.00)] cm extracts. Inhibition zone of Salmonella was showed significant different between the other concentrations of both extracts. Least MIC of Salmonella was 0.75 mg/ml for aqueous extract (Figure 3), however 0.25mg/ml for methanolic extract. MIC of
Klebsiella was 0.25 mg/ml for aqueous extract and methanol extract. Inhibition zone of klebsiella showed 1.6± (0.00), 2.0 ± (0.14) cm for 1mg/ml concentrations of aqueous and methanolic extract respectively. Protease was showed highest inhibition zone for 1mg/ml of aqueous extract when compared with positive control. There were was no significant different between the zones of inhibition obtained from Protease in the concentration of 1and 0.75 mg/ml methanolic extract and 0.75 mg/ml of aqueous extract and also no significant different between the 0.5mg/ml concentration of aqueous [1.35± (0.07)] and methanol [1.3± (0.00)]cm extracts. MIC of Protease was 0.25 mg/ml for aqueous extract and methanol extract. Least MIC of Bacillus was 0.75 mg/ml for aqueous extract whereas 1mg/ml for methanol extract. Zone of inhibition obtained from Bacillus for 1mg/ml concentration of aqueous and methanol extract was 1.55± (0.07), 1.45± (0.07) cm
respectively (Table 4).
4. DISCUSSION
Manoharan et al. 2014 also reported that phytochemicals screening in the pinattu showed positive results for steroids, triterpenoids, carbohydrates, saponin, flavonoids and proteins in varied amounts in the ethanolic and aqueous extracts while chloroform extract showed negative results for all tested compounds except for carbohydrate while glycoloids, alkaloids and tannins were not observed in any of the extracts. Most of these results agree with our qualitative analysis of pinattu.
Analysis of the total phenol and flavonoids content in plants materials is attracting thoughtfulness for pharmaceutical universal due to their beneficial effects on health of human and animal. Which are rich in phenolics are gradually being used in the food industry because these bioactive compounds prevent the lipids oxidative degradation and improve the quality and nutritive value of foods (Kähkönen et al. 1999). Phenolic compounds are one of the phytochemicals considered as secondary metabolites and these compounds derived from phenylalanine and tyrosine occurs ubiquitously in plants and is diversified (Naczk and Shahidi, 2004). In this experiment aqueous extract contained high amount of total phenolic content because which is in the plant depends on the type of extract, i.e. the polarity of solvent which are used in extraction. The solubility of the phenols is high in polar solvents and delivers high concentration of these compounds in the crude extracts during the extraction (Mohsen and Ammar, 2008).Palmyrah pinattu contained very less amount of flavonoids when compared with other tested phytochemical. These are
probably the most important natural phenols and one of the most diverse and general group of bioactive natural compounds. These compounds contained a broad spectrum of chemical and biological activities including antioxidant properties of radical scavenging properties. Several studies reported that bioactive components concentrations are affected by plant species and plant variety (Shiraiwa et al. 1991), degree of maturity, growing environment, agronomic factors such as climate and soil, cultivation year, location grown, season (Oleszek, 1996), and extraction method (Onning, 1993). For example saponins frequently are isolated by boiling in methanol (Oleszek et al. 1992), ethanol (Oleszek, 1990) and n- butanol (Massiot, 1992).
In our study methanol and aqueous extract contained better antimicrobial activity. Duddukuri et al., 2011 (Duddukuri, 1992) also reported that the antibacterial activity of methanol extract of Borassus flabellifer L. seed coat (soft outer shell) and examined against Gram positive bacteria i.e., Bacillus subtilis, Staphylococcus aureus and Gram negative bacteria i.e., Klebsiella pneumonia and Serratia marcescens. This showed consistently significant inhibitory activity. Furthermore, the minimum inhibitory concentration was ranged between 100μg to 1 mg/ml implying the significance of antibacterial activity.
5. CONCLUSION
This study was showed that among the four extracts, most of the biologically active phytochemicals were present in the aqueous and methanol extracts. Antibacterial activities with relatively lower MIC (≤ 1mg/ml) values, confirm that methanol and aqueous extracts of pinattu of Borassus flabellifer exhibit significant antibacterial activity when compared with other extracts. Therefore it could be considered as beneficial for further investigation on the palmyrah dried fruit pulp.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WP- Internal supervisor of the research project; SM- External supervisor; SS- Undergraduate research student; SS & SM carried out the antimicrobial activity study,
phytochemical studies and carried out statistical analysis; SS, SM &WP – Drafted the manuscript; SSV- coordinated & management of research activities. All authors read and approved the final manuscript.
Acknowledgments
Authors would like to express their gratitude to the staff of Palmyrah Research Institute, Jaffna, Sri Lanka, for their kind support in the project also kind support given by Ms.K.Robika, is greatly appreciated.
REFERENCE
Ariyasena DD, Jayasekera S, Jansz ER, Abeysekera AM. (2002). Effect of palmyrah (Borassusflabellifer L.) Fruit pulp on weight gain by mice. Vidyodaya J. Sci. 9: 99-105.
Avato P, Bucci R, Tava A, Vitali C, Rosato A, Bialy Z, Jurzysta M. (2006). Antimicrobial activity of saponins from Medicago spp. Structure-activity relationship. Phytother Res, 20:454-457.
Croteau R, Kutchan TM, Lewis NG., (2000). ‘Natural products (secondary metabolites)’. In: Buchanan, Gruissem, and Jones, (Ed.) Biochemistry & Molecular Biology of Plants. American Society of Plants Physiologists. Rockville: MD USA; pp: 1250-1318.
Duddukuri G R, Sastry YN, KaladharGK ,Rao KK and Chaitanya K K. (2011). Antibacterial activity of methanolic seed coat extract of Borassus flabelliferL. International J of Phamacecutical Sci and Res. 2: 2435-2438.
Gulcin_I, 2005. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 56, 491– 499.
Hadnick WF, Ahmad S, Pardini RS. (1998) Induction of oxidative stress by redox active flavonoids. Adv Exp Med Biol. 439: 131-150.
Hassan SM, Haq AU, Byrd JA, Berhow AM, Cartwright AL, Bailey CA. (2010). Hemolytic and antimicrobial activities of saponin-rich extracts from guar meal. J Food Chem, 119: 600-605.
Hiai S, Oura H, Nakajima T. (1976). Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. PlantaMedica. 29: 116–122.
Kähkönen MP, Hopia AI, Vuorela JH, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. (1999). Antioxidant activity of plant extracts containing phenolic compounds. J Agri Food Chem. 47, 3954–3962.
Manoharan N.A. Mahilrajan S. Srithayalan. S. (2014). Preliminary phytochemical screening of extracts of palmyrah fruit pulp. International Research Sessions. 18: p 239.
Massiot G, Lavaud C, Benkaled M, Le Men- Olivier L. (1992). Soya saponin VI’, a new matol conjugate from alfalfa and soybean. J Nat Prod. 55:1339-1342.
Mathers JC. (2002). Pulses and carcinogenesis: potential for the prevention of colon, breast and other cancers. Br J Nutr, 88: 273-279.
MauryaS and Singh D. (2010). Quantitative Analysis of Total Phenolic Content in Adhatodavasica Needs. Extracts International Journal of PharmTech Research. 4: 2403- 2406.
Mitra S, Dungan S. (1997). Micellar properties of Quillaja saponin. Effects of temperature, salt and pH on solution properties. J. Agric. Food Chem. 45: 1587-1595.
Mohsen MS, Ammar SMA. (2008). Total phenolic contents and antioxidant activity of corn tassel extracts. Food Chem. 112: 595-598.
Naczk M, Shahidi F. (2004). Extraction and analysis of phenolics in food. J Chromatogram, 1054: 95–111.
Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van NorrenK ,vanLeeuwen PA. (2001). Flavonoids: a review of probable mechanisms of action and potential applications. Am J ClinNutr, 74:418-25.
Oleszek W, Jurzysta M, Ploszynski M, Colquhoun IJ, Price KR, Fenwick RG. (1992). Zanhic acid tridesmoside and other dominant saponins from alfalfa (Medicago sativa L.) aerial parts. J Agric Food Chem, 40: 191-196.
Oleszek W, Price KR, Colquhoun IJ, Jurzysta M, Ploszynski M, Fenwick RG. (1990). Isolation and identification of alfalfa (Medicago sativa) root saponinsTheir activity in relation on a fungal bioassay. J Agric Food Chem. 38: 1810-1817.
Oleszek W. (1996). Alfalfa saponins Structure, Biological, Activity, and Chemotaxonomy. In: Waller GR and Yamasaki K, (Ed.) Saponins Used in Food and Agriculture. Plenum Press: New York; 155-170.
Onning G, Asp NG, Sivik B. (1993). Saponin content in different oat varieties and in different fractions of oat grain. Food Chem. 48: 251-254.
Ordonez AAL, Gomez JD, Vattuone MA and Isla MI. (2006). Antioxidant activities of Sechiumedule (Jacq.) Swart extracts. Food Chem. 97:452-458.
Rice-Evans CA, Miller NJ, PagangaG. (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci, 2, 152–159.
Sen A, And Batra A. (2012). Evaluation of antimicrobial activity of different solvent extracts of medicinal plant: meliaazedarach .International journal of current pharmaceutical research; 4, pp67-73.
Shiraiwa M, Harada K, Okubo K. (1991). Composition and structure of group B saponin in soybean seed. Agric. Biol. Chem. 55: 911-917.
Thabrew MI, Jansz ER. (2004). Nutritive importance of palmyrah products. Recent research development. Environ. Biol. 1: 43-60. Uluwaduge DI, Thabrew MI, Jansz ER. (2005). The effect of flabelliferins of the palmyrah fruit pulp on intestinal glucose uptake in ICR mice. J. Natn. Sci. Foundation. 4: 37-41.

